Publications from the Carcinogenic Potency Project
Gaylor, D.W., and Gold, L.S. Quick estimate of the regulatory
virtually safe dose based on the maximum tolerated dose for rodent
bioassays. Reg. Toxicol. Pharmacol., 22: 57-63 (1995).
PDF
With a limited subset of National Cancer Institute/National Toxicology Program
(NCI/NTP) bioassays, Gaylor (1989) showed that the regulatory
virtually safe dose (VSD), corresponding to an estimated lifetime
cancer risk of less than 10-6, could be estimated within
a factor of 10 simply by dividing the maximum tolerated dose (MTD),
estimated from the results of a 90-day study, by 380,000. The
purpose of this current study was to extend the analysis to all
carcinogens in the Carcinogenic Potency Database (CPDB) of Gold et
al. (1984, ’86, ’87, ’90, and ’93), utilizing the TD50 (average daily dose rate in
mg/kg body weight/day that was estimated to halve the probability
of remaining tumor-free at a specified tissue site throughout a
two-year study). Using the relationship between the upper bound on
the low-dose slope (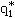 ) and the
TD50 reported by Krewski et al. (1993) and the ratio of
the maximum dose tested (Max-D)/TD50 obtained in our
present analysis, an estimate of the regulatory VSD was given by
the MTD/740,000, for NCI/NTP rodent carcinogens. This was about a
factor of two lower than the limited analysis conducted by Gaylor
(1989). There was little difference when the chemicals were divided
into mutagens and nonmutagens. Ninety-six percent (134 of the 139
NCI/NTP rodent carcinogens) of the regulatory VSDs calculated from
the individual TD50s obtained from the 2-year bioassays
were within a factor of 10 of the MTD/740,000. Gold et al. (1989)
investigated the distribution of the TD50 among
“near-replicate” experiments (where the same chemical
was tested more than once and was positive in the same strain, sex,
and species by the same route). The distribution of
TD50s from near-replicate experiments is similar to the
distribution of the Max-D/TD50. Hence, the estimate of
the regulatory VSD based on the Max-D/740,000, for NCI/NTP
bioassays, is about as precise as the estimate obtained from a
2-year bioassay. This questions the advisability of conducting a
2-year bioassay for purposes of regulatory risk estimation.
) and the
TD50 reported by Krewski et al. (1993) and the ratio of
the maximum dose tested (Max-D)/TD50 obtained in our
present analysis, an estimate of the regulatory VSD was given by
the MTD/740,000, for NCI/NTP rodent carcinogens. This was about a
factor of two lower than the limited analysis conducted by Gaylor
(1989). There was little difference when the chemicals were divided
into mutagens and nonmutagens. Ninety-six percent (134 of the 139
NCI/NTP rodent carcinogens) of the regulatory VSDs calculated from
the individual TD50s obtained from the 2-year bioassays
were within a factor of 10 of the MTD/740,000. Gold et al. (1989)
investigated the distribution of the TD50 among
“near-replicate” experiments (where the same chemical
was tested more than once and was positive in the same strain, sex,
and species by the same route). The distribution of
TD50s from near-replicate experiments is similar to the
distribution of the Max-D/TD50. Hence, the estimate of
the regulatory VSD based on the Max-D/740,000, for NCI/NTP
bioassays, is about as precise as the estimate obtained from a
2-year bioassay. This questions the advisability of conducting a
2-year bioassay for purposes of regulatory risk estimation.
Since resources are available to test only a small fraction of
chemicals to which humans are exposed, a preliminary estimate of
the regulatory VSD can be useful in setting testing priorities. If
the expected human exposure level is below the regulatory VSD
estimated from the MTD, a chemical may be assigned low priority for
testing in a 2-year bioassay. Based on the work of Rulis (1986,
’89, and ’92), the FDA (1993) proposed a
“threshold of regulation” of a dietary concentration of
0.5 ppb for substances used in food-contact articles. Rather than
setting a single dietary concentration (0.5 ppb) for the threshold
of regulation for all substances, the results of the present
analysis could be used to make the procedure more chemical specific
based upon the MTD.
The high correlation between the MTD and estimate of cancer
potency (TD50) can be exploited to provide a
preliminary, hypothetical upper bound estimate of cancer risk for
exposure to a chemical without conducting a 2-year animal bioassay.
Thus, the expected level of human exposure relative to the MTD can
be used to determine the priority for further research on a
chemical, such as mechanistic studies.
Return to the Carcinogenic Potency Project
Home Page: 
Last updated: August 6, 2007
PDF documents are best viewed with the free Adobe® Reader
http://get.adobe.com/reader
Excel documents are best viewed with the free Excel® Viewer
http://www.microsoft.com/en-us/download/details.aspx?id=10
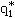 ) and the
TD50 reported by Krewski et al. (1993) and the ratio of
the maximum dose tested (Max-D)/TD50 obtained in our
present analysis, an estimate of the regulatory VSD was given by
the MTD/740,000, for NCI/NTP rodent carcinogens. This was about a
factor of two lower than the limited analysis conducted by Gaylor
(1989). There was little difference when the chemicals were divided
into mutagens and nonmutagens. Ninety-six percent (134 of the 139
NCI/NTP rodent carcinogens) of the regulatory VSDs calculated from
the individual TD50s obtained from the 2-year bioassays
were within a factor of 10 of the MTD/740,000. Gold et al. (1989)
investigated the distribution of the TD50 among
“near-replicate” experiments (where the same chemical
was tested more than once and was positive in the same strain, sex,
and species by the same route). The distribution of
TD50s from near-replicate experiments is similar to the
distribution of the Max-D/TD50. Hence, the estimate of
the regulatory VSD based on the Max-D/740,000, for NCI/NTP
bioassays, is about as precise as the estimate obtained from a
2-year bioassay. This questions the advisability of conducting a
2-year bioassay for purposes of regulatory risk estimation.
) and the
TD50 reported by Krewski et al. (1993) and the ratio of
the maximum dose tested (Max-D)/TD50 obtained in our
present analysis, an estimate of the regulatory VSD was given by
the MTD/740,000, for NCI/NTP rodent carcinogens. This was about a
factor of two lower than the limited analysis conducted by Gaylor
(1989). There was little difference when the chemicals were divided
into mutagens and nonmutagens. Ninety-six percent (134 of the 139
NCI/NTP rodent carcinogens) of the regulatory VSDs calculated from
the individual TD50s obtained from the 2-year bioassays
were within a factor of 10 of the MTD/740,000. Gold et al. (1989)
investigated the distribution of the TD50 among
“near-replicate” experiments (where the same chemical
was tested more than once and was positive in the same strain, sex,
and species by the same route). The distribution of
TD50s from near-replicate experiments is similar to the
distribution of the Max-D/TD50. Hence, the estimate of
the regulatory VSD based on the Max-D/740,000, for NCI/NTP
bioassays, is about as precise as the estimate obtained from a
2-year bioassay. This questions the advisability of conducting a
2-year bioassay for purposes of regulatory risk estimation.